Kjeldahl Apparatus solution|estimation of nitrogen by kjeldahl method : agent The Kjeldahl method uses sulfuric acid, a variety of catalysts, and salts to convert organically bound nitrogen in samples to ammonium with its . 29 de jan. de 2022 · 近日,网石(Netmarble)在韩国首尔举办媒体记者会,揭露未来游戏阵容与公司方向,同时公布了知名网游《RF Online》IP所开发的衍生科幻机甲射击MMORPG新作《RF Project》,未来将登陆PC和手机平台,发售日期待定。
{plog:ftitle_list}
WEB6 de dez. de 2019 · I am also happy to review your VR experience and provide any feedback / insights I can provide. You can also checkout my website: .
The Kjeldahl method may be broken down into three main steps: Digestion - the decomposition of nitrogen in organic samples utilizing a concentrated acid solution. This is accomplished by .The Kjeldahl method or Kjeldahl digestion in analytical chemistry is a method for the quantitative determination of a sample's organic nitrogen plus ammonia/ammonium. (NH3/NH4 ). Without modification, other forms of inorganic nitrogen, for instance nitrate, are not included in this measurement. Using an empirical relation between Kjeldahl nitrogen and protein, it is an important method for indirectly quantifying protein content of a sample. This method was developed by Johan KjeldahlThe steps include digestion, distillation, and titration. 1. Digestion: In this method, a certain substance or sample is heated in the presence of sulphuric . The Kjeldahl method uses sulfuric acid, a variety of catalysts, and salts to convert organically bound nitrogen in samples to ammonium with its .
Total Kjeldahl Nitrogen (TKN) analysis determines both the organic and the inorganic forms of nitrogen. The analysis starts with an acid digestion of the sample organics, converting organic nitrogen to ammonia. The Kjeldahl method may be broken down into three main steps: digestion, distillation, and titration. Digestion is accomplished by boiling a homogeneous sample in .
The Kjeldahl method uses sulfuric acid, a variety of catalysts, and salts to convert organically bound nitrogen in samples to ammonium with its subsequent measurement (Sáez-Plaza et al., 2013).In 1883, Johan Kjeldahl introduced his "New Method for the Determination of Nitrogen in Organic Bodies", revolutionising nitrogen analysis and setting new standards. Since then, the method has become indispensable in areas such .
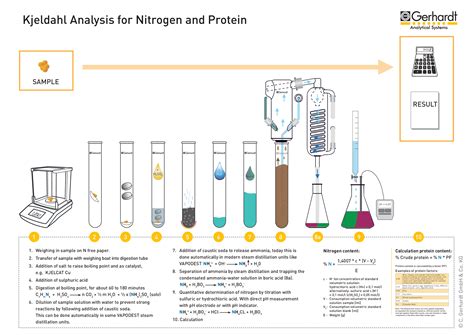
Three basic steps are required to perform the Kjeldahl analysis: Conversion of nitrogen in the sample to ammonium sulfate. Separation of the ammonium ion. Quantitative determination of . Three basic steps are required to perform the Kjeldahl analysis: Conversion of nitrogen in the sample to ammonium sulfate. Separation of the ammonium ion. Quantitative determination of the ammonium ion and . The Kjeldahl method was introduced in 1883 and consists of three main steps: sample digestion, distillation, and ammonia determination (titration being the primary method).
Kjeldahl Method Apparatus and Equipment. . The solution is now distilled, and a little amount of sodium hydroxide is added to help turn the ammonium salt into ammonia. The distilled vapours are then contained in an . The Kjeldahl method has been validated and standardized for total (crude) protein estimation for a wide variety of food matrices, indirectly determined by their nitrogen content, and is the reference method adopted by .Kjeltec™ 9 Autosampler 20, 1 rack with 20 Kjeldahl tubes 250 ml or 400 ml . Kjeltec™ 9 Autosampler 60, 3 racks with 20 Kjeldahl tubes 250 ml or 400 ml. Storage capacity: 200 batches in the instrument software. Analysis time: 3.5 minutes at 30 mg N (6.5 minutes at 200 mg N) Distillation capacity ~ 40 ml/min: Measuring range: 0.1 - 210 mg N .
Figure 5.10 shows a modified Kjeldahl distillation apparatus which was successfully used by Weakley in 1936 [].It has the peculiarity that metal heads take the place of the usual glass connecting bulbs. The block-tin condenser tube which comes with this particular apparatus is the right length to be soldered into the head and should extend to within 2 or 3 mm of the top .The VELP solution VELP Scientifica has developed the widest range of Kjeldahl apparatus consisting on digestion units (traditional or automatic models) and four distillation units with multiple innovations and features that distinguish them from conventional instruments, to respond to the different needs in R&D, QC and QA laboratories today.
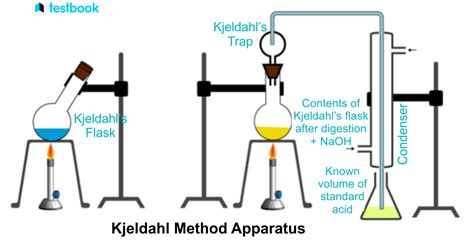
Kjeldahl method, in analytical chemistry, procedure widely used for estimating the nitrogen content of foodstuffs, fertilizers, and other substances, invented in 1883 by a Danish chemist, Johan G.C.T. Kjeldahl. The method consists essentially of transforming all nitrogen in a weighed sample into ammonium sulfate by digestion with sulfuric acid, alkalizing the solution, and .
The Kjeldahl process is an established method which is widely used for all kinds of food samples but also for environmental, chemical and pharmaceutical samples. It involves a digestion step to decompose proteins and other nitrogen-containing species and is followed by steam distillation to isolate the ammonia for nitrogen quantification.Micro Kjeldahl Apparatus offered by Labconco include Micro Digestors and RapidStill I. Fully assembled and ready to use, Micro Digestors provide accurate results for Kjeldahl digestions using either 30 or 100 milliliter flasks. It is the perfect companion to the RapidStill I, which is designed for laboratories that are determining micro .Labconco Corporation 8811 Prospect Avenue Kansas City, MO 64132-2696 (816) 333-8811 phone (816) 363-0130 fax (800) 821-5525 toll-free
protein estimation by kjeldahl method
Kjeldahl method procedure. Kjeldahl nitrogen method is a recognized technique that is covered in the “main three procedures.” 1. Digestion: The given or collected organic sample is first treated with a strong acid solution, primarily H 2 SO 4. At a very high temperature, the solution is brought to a boil.
Kjeldahl Nitrogen Primary Standard Set set of 3 2277800 Nitrogen Ammonia Standard Solution, 1.0-mg/L NH3–N 500 mL 189149 Nitrogen, Ammonia Standard Solution, 10-mL Voluette Ampules, 150 mg/L 16/pkg 2128410 Wastewater Influent Standard Solution, Mixed Parameter, for NH3-N, NO3-N, PO4, COD, SO4, TOC 500 mL 2833149 Optional reagents and apparatus The boric acid solution is to be poured into a conical flask. Set the flask on the distillate collection apparatus. Set up all other apparatus with the distillation unit. Mix the digested sample by rotating. Transfer 10ml of the digested sample into a distillation flask. Add 50ml of 40% sodium hydroxide at this stage. Alkalizing the solution liberates ammonia which is quantitatively steam-distilled and determined by titration. BUCHI introduced its first Kjeldahl system in 1961 with a simple, but first automatic nitrogen determination apparatus with integrated steam generator.
Hooded Combination Kjeldahl units enclose the distillation and digestion apparatus so that process heat may be contained and vented from the laboratory. The hood surrounding the apparatus includes three collars: one .
(for the high percentage that both fractions represent) or Kjeldahl N (KjN), and are measured using the Kjeldahl method. This method includes a wet digestion of the soil sample to mineralize the N to NH 4, which will be distilled and measured. The inorganic forms of N (NH 4 +, NO 3-and NO 2-) are generally determined by distillation or colorimetryOn cooling, the solution is diluted with water and the ammonia is liberated by using the micro-Kjeldahl distillation apparatus shown in Fig. 5. In operation, the ammonium bisulfate solution in the micro-Kjeldahl digestion flask is transferred through funnel B to the bottom of distilling flask D, followed by 8 mL of 30% sodium hydroxide solution.Kjeltec™ 9 Autosampler 20, 1 rack with 20 Kjeldahl tubes 250 ml or 400 ml . Kjeltec™ 9 Autosampler 60, 3 racks with 20 Kjeldahl tubes 250 ml or 400 ml. Storage capacity: 200 batches in the instrument software. Analysis time: 3.5 minutes at 30 mg N (6.5 minutes at 200 mg N) Distillation capacity ~ 40 ml/min: Measuring range: 0.1 - 210 mg N .The Kjeldahl Method, The Digestion Phase, Distillation and Titration, VELP Kjeldahl Systems, analytical instruments, elemental analyzers, digesters, extractors, magnetic stirrers, laboratory instruments. . To quantify the amount of ammonia in the receiving solution. The amount of nitrogen in a sample can be calculated from the quantified .
The conversion of organic nitrogen to ammonium sulfate is achieved by digesting the sample with concentrated sulfuric acid. It is basically here where Kjeldahl succeeded over the Varrentrapp–Will method: while the recovery of nitrogen in an alkaline media was incomplete for many types of samples, the acidic media showed a much higher yield, closer to the actual .The Kjeldahl Method The basis of the Kjeldahl method is the conversion of organic nitrogen into ammo-nium sulfate, and subsequent separation and determination of the ammonium ion. Although initially developed by Johan Kjeldahl to determine nitrogen content in proteins, many further modifications were made to the method by different .
The solution is made alkaline and the ammonia is determined by distilling into an excess of standard acid, followed by titrating the excess acid. . requirements of the Kjeldahl apparatus available to them. Glassware should be carefully inspected for defects before use. Dispose of spent copper and selenium
5H2O in 1 liter may be used.) 6. NaOH, pellets or solution, nitrate-free. For solution .Kjeldahl nitrogen determinations are performed on a variety of food substances. The Kjeldahl method may be broken down into three main steps: digestion, distillation, and titration. 1. Digestion: is accomplished by boiling a homogeneous sample in concentrated sulphuric acid. The end result is an ammonium sulphate solution. 2.
kjeldahl method diagram
Including a 500 ml Kjeldahl flask, multiple adaptors, a 50 ml dropping funnel, a splash head, and a Liebig condenser, this apparatus with 19/26 joints offers a comprehensive setup for efficient Kjeldahl distillation processes in laboratory applications.Overcoming the original testing sensitivity issues, the Kjeldahl distillation systems conduct nitrogen analyses that determine the total .
Kjeldahl, Distillation Apparatus, 2-Unit, 115V Shop Labconco Kjeldahl, Distillation Apparatus, 2-Unit, 115V at Thomas Scientific, your trusted partner in Science. Skip To Main ContentClassical Macro-Kjeldahl Apparatus. Many governmental and regulating agencies have developed methodologies that specify the classical macro-Kjeldahl apparatus. For example, standard methods for low level nitrogen determinations in water (0-10 mg/1) require a sample size of 250 to 500 ml, and therefore large Kjeldahl flasks.

NFL Odds Today - NFL Spreads, Lines, and Moneylines. At FanDuel Sportsbook, we provide a user-friendly platform for football fans eager to bet on NFL odds to win each game in the 2025 NFL season. Our online sportsbook offers a wide range of betting options, including NFL game lines, Super Bowl odds, various NFL prop bets, and futures.
Kjeldahl Apparatus solution|estimation of nitrogen by kjeldahl method